Explain How Alkali Metals Are Different From Noble Gases
They tend to lose 1 electron and attain stability by forming cations. Valence electrons are different metals have a full shell while noble gases have less than half a shell.
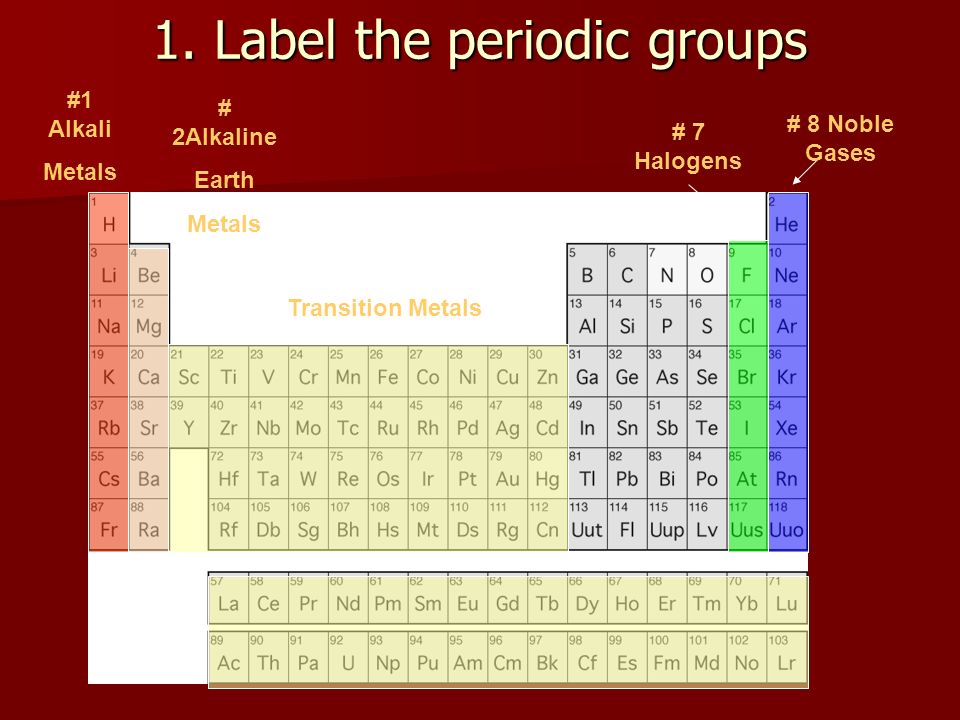
Unit 3 Review Chemistry 1 Alkali Metals 2alkaline Earth Metals Transition Metals 7 Halogens 8 Noble Gases 1 Label The Periodic Groups Ppt Download
The elements of Group 1 alkali metals and Group 2 alkaline earth metals which have ns1 and ns2 outermost electronic configuration belong to the s-Block Elements.
. Alkali metals react with water to form basic hydroxides and liberate hydrogen. The s block includes the first two groups alkali metals and alkaline earth metals as well as hydrogen and helium. Increase Decrease or Constant 4 Explain exceptions to the overall trend based on electron configuration.
React strongly with water. Can cut with a knife. The noble gases all have low boiling points.
Alkali metals are simply metals of the group 1 elements because they 1 valence electron. The boiling points of these alkali metals show a similar pattern to the melting points. The reaction of Alkali Metal with Water.
React most readily with halogens. Noble gases are highly stable. The elements in group 0 are called the noble gases.
Alkali metal floats on the. They are hence chemically reactive. A Noble gases have a full shell of valence electrons while metals have only a few valence electrons.
As both Alkali metals and alkali earth metals are the first two groups in the periodic table the difference between alkali metals and alkaline earth metals is a subject of. Alkali metals are group 1 elements with one valence electrons. Osea que un alkali metal es un metal alkalino y los noble gases son un tipo de gases.
They are all reactive metals with low ionization enthalpies. The elements in group 1 are known as alkali metals. Reactivity increases as you move down the group 6.
Explain why noble gases are inert and do not form ions. Your answer will be Alkali MetalsBecause the alkali metals make up Group 1 of the table and comprise lithiumLi through franciumFr. Noble gas on the other hand.
Noble gases are widely used in different fields from incandescent lighting to excimer lasers. Secondly on the periodic table the alkali metals are group. The alkali metals group 1A in the periodic table differ from the noble gases group 18 in the periodic table in several ways The alkali metals are soft shiny highly reactive elements that form colored solutions when they react with water Their highest oxidation state is 1 and their electrons are transferred to form cations They have high first ionization energies which make.
The alkali metals consist of the chemical elements lithium Li sodium Na potassium K rubidium Rb caesium Cs and francium Fr. See full answer below. The noble gases are all chemically unreactive which means they are inert.
This shared electron configuration results in their having very similar characteristic. The key difference between alkali metals and alkaline earth metals is that all alkali metals have an electron in their outermost shell whereas all the alkaline earth metals have two outer electrons. Hydroxides of Alkali Metals.
How are the noble gases different from other non metals. A Alkali metals have one electron in their outer View the full answer. The alkali metals are the elements located in Group IA of the periodic table are a lot different from the noble gases also known as the inert gases are located in Group VIII of the periodic table.
Use the appropriate factors to explain the overall trend indicated by the dark line and the exceptions to it1 Effective Nuclear energy. These elements are soft metals electropositive and form basic oxides. Explain how alkali metals are different from noble gases.
Group 1 elements form alkaline solutions when they react with water which is. The reaction of the metal is exothermic and the enthalpy increases from lithium to cesium. When the element with the atomic number of 119 is discovered what group will it be in.
Correct answer to the question Explain how alkali metals are different from noble gases. Together with hydrogen they constitute group 1 which lies in the s-block of the periodic tableAll alkali metals have their outermost electron in an s-orbital. First of all alkali metals are usually solid while the noble gases are in gaseous form.
Noble gases are group 18. The metals become more reactive as. Compare and contrast ionization energy and atomic radius.
En la foto de la tabla periodica los de a izquierda del todo son los ALKALI METALS y los de la derecha del todo son NOBLE GASES. They must be stored under oil to keep air and water away from them. Alkali metals are the most reactive METALS and the noble gases are unreactive GASES.
Since the alkali metals react with nitrogen oxygen and water in the air they are always stored under kerosene. They belong to the right-hand column in the periodic table. Increase Decrease or Constant2 Shielding.
They react rapidly very fast with water producing an alkaline solution and hydrogen gas. Make an argument for placing hydrogen in the halogen group rather than the alkali metals. Alkali metals react with water to form alkalis hence they are.
The p block includes the last six groups Groups 13 to 18 and contains among others all of the metalloids and nonmetals. Many of the physical properties common to metals. Increase Decrease or Constant3 Atomic Radius.
What is the difference between noble gases and metals. Metals have few enough valence electrons that the energy required to remove electrons from their atoms and form ions with their noble gas configuration is less. Only one valence e-.
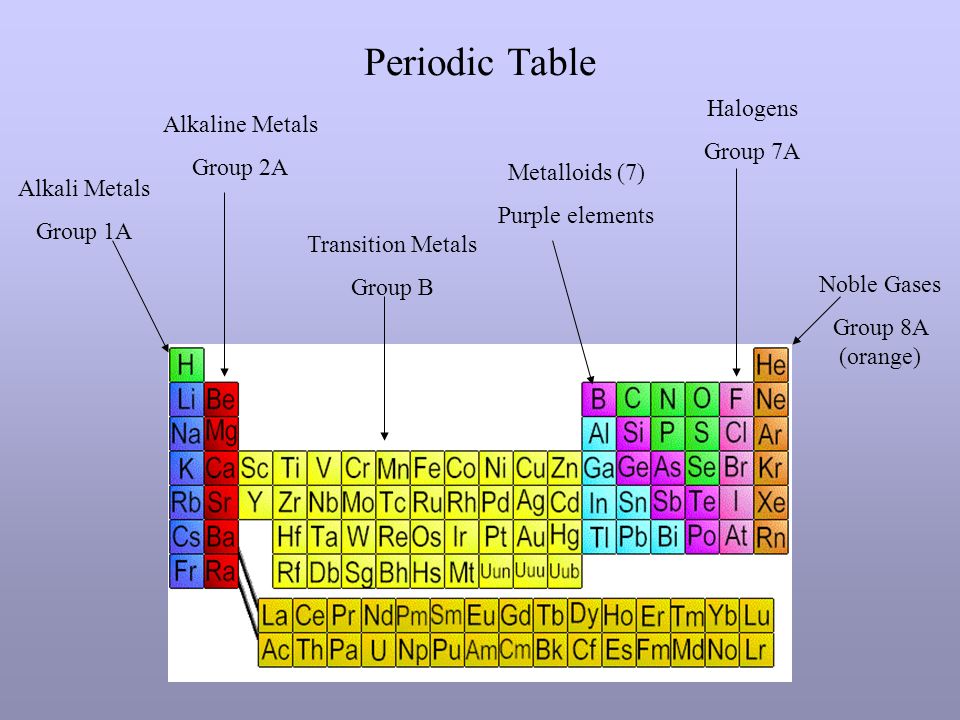
Periodic Table Alkali Metals Group 1a Alkaline Metals Group 2a Transition Metals Group B Metalloids 7 Purple Elements Halogens Group 7a Noble Gases Group Ppt Download
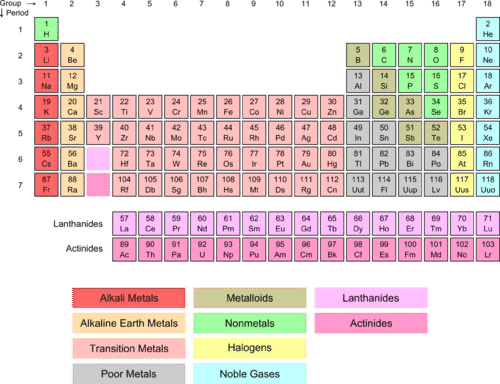
What Is The Difference Between Alkaline Metals And Alkaline Earth Metals Quora
No comments for "Explain How Alkali Metals Are Different From Noble Gases"
Post a Comment